|
 Why Keeping Sterilizer Record Keeping Is Important Why Keeping Sterilizer Record Keeping Is Important


|
eogas
|
Do I need record keeping for sterilized instruments?
Is record keeping for sterilization cycles required?
|
|
|
|
|
Record keeping is a critical component of any sterilization monitoring protocol. The Centers for Disease Control and Prevention (CDC), the American Dental Association (ADA), and the Organization for Safety and Asepsis Procedures (OSAP), all recommend keeping a log for the purpose of recording daily sterilization records.
Record keeping is a legal responsibility for every practice and should be maintained in a proper manner. Per AAMI: “Ideally, every reprocessed medical device, especially and implant, should be fully traceable to the patient on whom it is used…recording the sterilizer load identifier on the patient chart”.
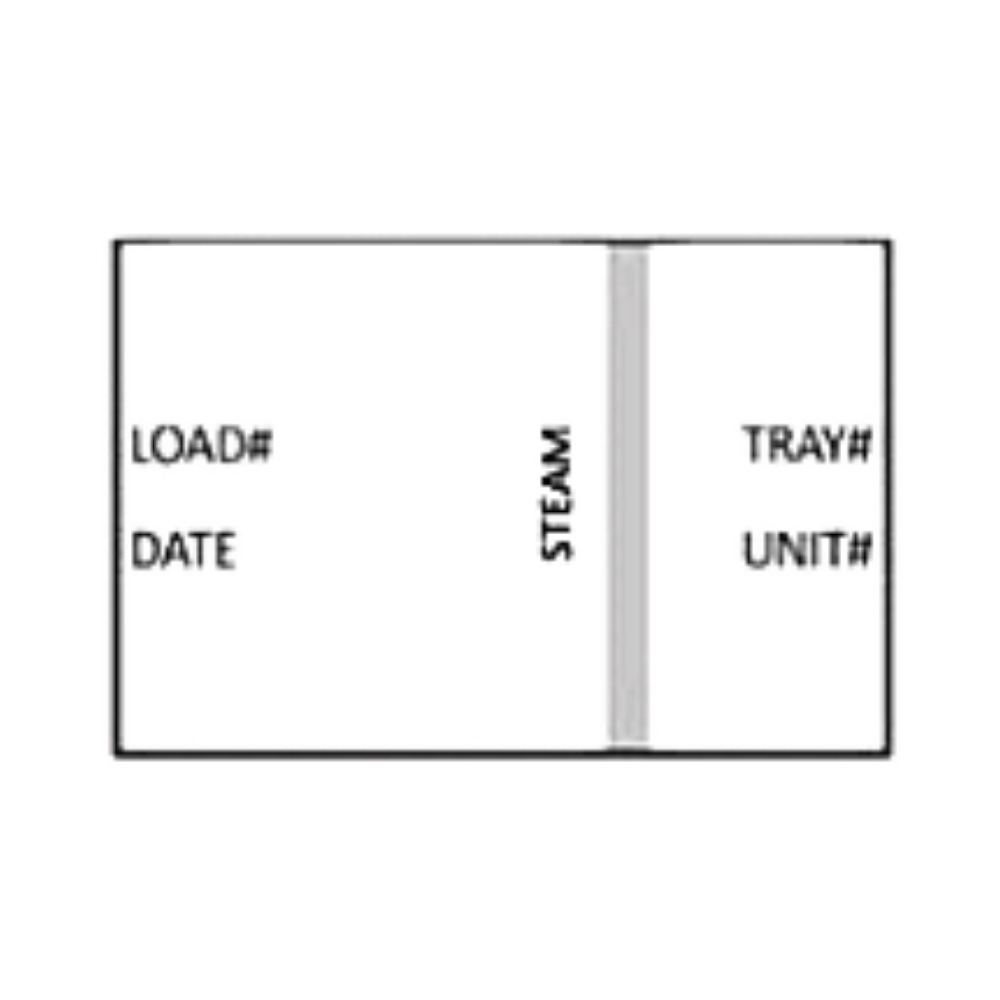
Per AORN: “Documentation of cycle information and monitoring results should be maintained in a log (electronic or manual) to provide tracking to the individual patient.” Each item or pack should be labeled with a lot or load control number to include:
- Identify the sterilizer Load or Cycle number
- Date of processing
- Sterilizer identification number
- Expiration date
Protect your patients, staff, and yourself. Reduce risk liability and protect your practice. If your sterilizer fails, know which instruments to recall. Efficiently. Duraline Systems recommend the Labelex™ I.D. labeling system, to provide traceability of every sterilized item.
|